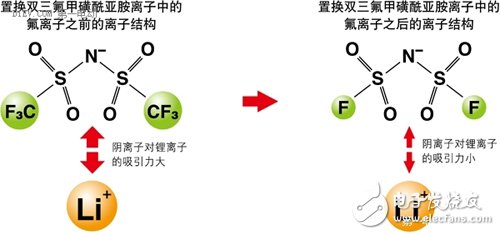
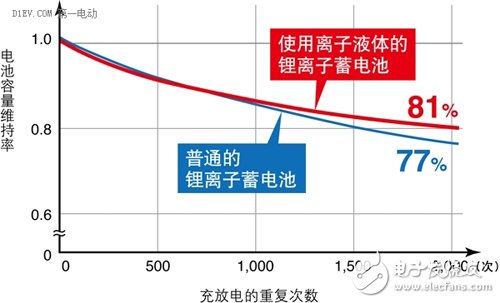
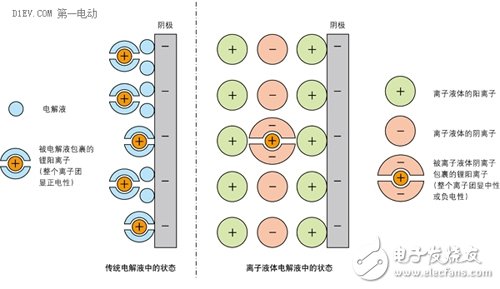
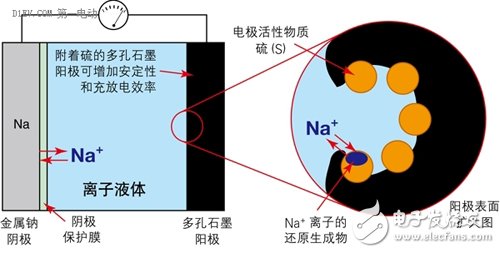
First, all kinds of battery related accidents Case 1 On January 7, 2013, a fire broke out on a Japanese Airways JA829J flight at Boston International Airport, which was operated by a Boeing 787. The fire source is the Auxiliary Power Unit (APU) on the Boeing 787. The Boeing 787 is the latest passenger aircraft from Boeing. The use of a large number of composite materials, with low fuel consumption rate and less pollutant emissions. At the same time, the cabin adopts new technologies such as LED lighting that can adjust brightness and light color, and large-area portholes that adjust transparency by electronic means; it has low noise, low maintenance cost, high reliability, and a medium-sized body capable of flying long distances. Excellent performance of the route. Whether for airlines or passengers, it is a true "Dream liner" commensurate with its model name. After an intermediate investigation report issued by the National Transportation Safety Board (NTSB), the aircraft auxiliary power unit was seriously burnt. It can be judged that the accident was caused by the overheating of the battery pack and caused the fire to burn. Fortunately, the accident happened on the ground, so it did not cause a bigger disaster. At the same time, because the body and battery pack are completely preserved, sufficient conditions are also provided for investigating the cause of the accident. Case 2 On April 26, 2015, in a power station in Shenzhen, the bus was burned into a skeleton due to a fire in the battery pack inside the bus. According to "First Electric Network" report: the direct cause of the accident is: after the vehicle's power battery is fully charged, the power battery is overcharged for 72 minutes, and the overcharge is 58kWh, causing multiple battery boxes to have thermal power out of control and electrolyte leakage. Short circuit, resulting in fire. Case three In the early morning of July 22, 2015, a fire broke out in a parking lot in Xiamen. A total of 11 buses were burned at the scene, and many of them were almost burned into skeletons. According to reports: The cause of the fire was self-ignition caused by electrical failure of the battery pack at the rear of the bus. Six of the 11 fired buses were hybrid buses. Since 2010, in the domestic new energy vehicle-related accidents, accidents caused by battery safety problems have occurred almost every year, which has raised concerns about the safety of this new type of vehicle to a certain extent. According to reports: In the second phase of the car purchase index lottery activity held in Beijing this year on April 26, 2015, the number of new energy passenger car index applications is smaller than the current target quota, no need to shake the number, directly configured. Second, the soft rib of the battery When the ordinary battery is discharged, the anode undergoes an oxidation reaction, and the generated electrons are led out by an external circuit and used by the current in the form of a current; the current flows through the circuit to the cathode, and the cathode obtains an electron reduction reaction. The difference between a rechargeable battery and a normal battery is that when the external voltage is higher than the voltage that the battery can provide, the anode and cathode inside the battery will react to the opposite reaction at the time of discharge, that is, the anode material is reduced and the cathode material is oxidized. In order to achieve the effect of charging. In the process in which the electrode of the battery undergoes an oxidation reaction and a reduction reaction, the electrolyte provides a passage through which ions generated by the reaction pass, and thus plays an important role in the battery. Generally, an aqueous solution or an organic solvent can be used as the electrolyte in the battery. However, when the type of battery is a lithium ion battery, the aqueous solution cannot be used due to the following two main reasons: 1. The main substance (such as lithium sheet) that constitutes the electrode will react with water. If there are too many side reactions in this category, it will accelerate the attenuation of battery capacity; 2. The theoretical decomposition voltage of water is 1.23v, so the highest voltage of the battery with the aqueous solution as the electrolyte system can only reach about 2.0v (such as lead-acid battery). Therefore, the electrolyte used in the battery is basically an organic solvent. However, the biggest weakness of batteries using organic solvents is that they are easy to burn. At present, the organic solvents used in the batteries have the characteristics of being easily volatilized. When the battery is heated for some reason, it will accelerate the volatilization of the organic solvent, and the volatilized gas will be easily burned, which will cause an explosion if certain conditions are met. This is probably the root cause of the burning accidents of the various new energy vehicle batteries mentioned above. In other words, if a liquid can be found that is not easily vaporized, then a battery burning accident can be eliminated. From another perspective, we can see that the battery used in large vehicles has a very high probability of failure. An ordinary battery module has a specification of 100AH/3.2V. If it is used to drive a car, the driving voltage is about 72-150 volts, and it is necessary to combine 20-30 such battery modules. If used to drive a bus, the driving voltage is about For 200-500 volts, you need to combine 70-170 such battery modules! Even if the pass rate of each battery module is as high as 99.9%, the battery pack consisting of 170 batteries has an overall pass rate of only 84%! In other words, if a bus fleet holds 100 bus buses, there may be more than a dozen of them that have battery burning accidents! We know that there are only two kinds of liquid substances (excluding liquid elements) that humans often come into contact with at normal temperature: water (and its solution) and organic solvents. As can be imagined from the above description, if the conventional electrolyte made of these two liquids is limited, the development of the battery has encountered a bottleneck. Third, found a new type of liquid In this situation, a new type of liquid, Ionic Liquids, has entered the researcher's line of sight. Ionic liquids are liquid salts (salt "salt" in chemical classification, which is different from "salt" in daily life). In general, a salt that is liquid below 100 degrees Celsius (also classified as 150 degrees Celsius or less) is called an ionic liquid. Among them, an ionic liquid which is in a liquid state especially at a normal temperature and a normal pressure is called "RoomTemperature Ionic Liquid" (RTIL). In fact, ionic liquids were discovered in the 1950s. However, because it did not find a better combination of stability at the time, it was shelved. In the 1990s, with the development of batteries with high storage capacity, ionic liquids were again noticed in order to find new and better electrolytes. At the same time, with the deepening of research, while the various samples are constantly being produced, the mass production technology of ionic liquids is gradually established, and it is called "fantastic new materials." Different ionic liquids can be obtained by changing the combination of anions and cations of the ionic liquid. At present, there are about 1,300 kinds of papers published. But in theory, there are about 10^18 ionic liquids. The biggest feature of ionic liquids is that they have many advantages that are not found in aqueous solvents and organic solvents: 1. The ionic liquid has a high ionic conductivity; 2. In a relatively large temperature range (-30 ° C ~ +300 ° C) can maintain a stable liquid state, heat resistance is very strong; 3. It has a low vapor pressure and thus has a non-flammable property; 4. The general ionic liquid is non-volatile, so separation and reuse after chemical reaction are relatively easy. An environmental substance or catalyst that can be used as a chemical reaction; 5. The viscosity of the ionic liquid is low; 6. Under certain combinations of anions and cations, ionic liquids cannot dissolve with water or organic solvents. When it is mixed, phase separation occurs. So in some cases, the ionic liquid is called the "third liquid." From the various characteristics of the ionic liquid, it can be said that it is a chemical solution of the battery. Fourth, foreign research on ionic liquids for storage batteries It is not acceptable to replace the electrolyte of the battery with an ionic liquid. On the one hand, the chemical bond between the anion and cation of the ionic liquid is relatively strong, which limits the charge movement in the electrolyte; on the other hand, the decomposition reaction of the ionic liquid occurs on the anode and cathode surfaces of the battery, resulting in the electrode and the ionic liquid. Creating an interface between them also hinders the flow of electrons. As a result, compared with the lithium ion battery currently in use, the battery using the ionic liquid has a lower power output density (W/kg) and the performance of the battery itself is also lowered. In response to these problems, the current research on ionic liquids in foreign countries mainly focuses on the following aspects. 1. Change the electrochemical properties of ionic liquids The essence of ionic liquids is a liquid substance in a perfectly balanced state composed of very complex anions and cations. Therefore, as long as the equilibrium state can be maintained without being destroyed, a certain portion of the anion and cation can be replaced, thereby achieving the purpose of changing the electrochemical characteristics of the ionic liquid. Professor Ishikawa Masashi of Kansai University in Japan studied the ionic liquid used as a lithium ion battery electrolyte, and found that the anion has a very high attraction to lithium ions, so that lithium ions can hardly move in the ionic liquid. As a result, lithium ion batteries using ionic liquids are completely incapable of functioning. Under this circumstance, Professor Ishikawa reduced the original three fluorine atoms in the anion to one, which greatly reduced the binding force of the anion to lithium ions, and successfully developed a new type of lithium ion battery. Figure 1 changes the composition of an ionic liquid to change its electrochemical properties. Ionic liquids are compounds composed of cations and anions. It is precisely because these anions and cations are closely connected to each other that the vapor pressure of the ionic liquid is low. Therefore, the key to studying ionic liquids is how to minimize the attraction of anions and cations, especially the attraction of anions to metal ions (eg, lithium ions), while maintaining the electrochemical and physical properties of ionic liquids. . In this way, the disadvantages of the conventional lithium ion battery can be avoided while utilizing the characteristics of the ionic liquid. Figure 2 shows the charge and discharge performance of a battery using an ionic liquid higher than that of a conventional battery. As a symbol of success in ionic liquid batteries, on June 20, 2014, the battery developed by Professor Ishikawa was installed on the ultra-small artificial earth satellite "Hodoyoshi No. 3" developed by the University of Tokyo, Japan, and experiments were conducted in space. Up to now, batteries installed on satellites have been made of a volatile material and have been deteriorated by cosmic rays. Therefore, in order to ensure safety, a strong outer casing must be provided, so that the weight is relatively large. The lithium-ion battery using ionic liquid developed by Professor Ishikawa, because of the use of ionic liquids, has no problem of volatilization and performance changes, so it can reduce the weight of many satellites. In addition, the ultra-small satellite "Hodoyoshi No. 3" is not suitable for the conventional large-sized batteries. Therefore, the use of ionic liquid batteries is a new good news for ultra-small satellites. Figure 3: Ionic liquid battery installed on the satellite (capacity: 1Ah, maximum voltage: 4.2V) Figure 4: Ultra-small satellite "Hodoyoshi 3" with ionic liquid storage battery installed According to Japanese media reports, the experiments carried out using the battery developed by Professor Ishikawa have achieved a complete success, which undoubtedly pointed out a new path for the development of ionic liquid batteries and aerospace technology. 2. Study new electrodes that adapt to the electrochemical properties of ionic liquids Ionic liquid electrolytes have a large effect on the electrode materials of conventional batteries. This effect is divided into two parts: (1) Ionic liquids have a destructive effect on the electrode material In the research of traditional batteries, it was found that the use of silicon-based cathode materials can greatly increase the reversible capacity of the cathode (up to 4,200 mAh/g). However, like conventional batteries, ionic liquids also pulverize the silicon-based electrodes. For graphite electrodes, ionic liquids also cause turbulence in the layered structure of the graphite molecules, thereby reducing battery performance. In order to solve this problem, researchers such as Prof. Shinichi, Professor of Tokyo University of Science and Technology, used functional adhesives such as polyacrylic acid in ionic liquid batteries to successfully improve battery capacity and charge and discharge life. As a result, a battery with an energy density of 600 Wh/kg (300-360 Wh/kg for a conventional battery) was successfully developed, and the charge and discharge life has also been greatly improved. (2) The ionic liquid forms an interface around the electrode that blocks the ion-conducting charge The ionic liquid consists only of two kinds of cations and anions. In the ionic liquid, the lithium ions existing as cations are surrounded by the anions of the ionic liquid, and the entire ion group is negative. At the same time, the cation of the ionic liquid is surrounded by the cathode of the battery. Surrounded by. These two effects cause the lithium ions in the battery to be inaccessible to the cathode and fail to form an internal current loop. Figure 5 is a schematic diagram of the formation of a barrier ion conduction interface around the electrode in the ionic liquid To solve this problem, researchers at Keio University in Japan use an ionic liquid with a lower affinity for lithium ions. When the ion group encapsulating lithium ions approaches the cathode, lithium ions are released from the ion group. As a result, the strength of the interface around the electrode can be weakened. 3. Using new electrode materials In the research of new batteries using ionic liquids, Na-S batteries have once again attracted attention. The main reasons are as follows: (1) The theoretical capacity of sulfur is up to 1,670 mAh/g, which is more than 10 times the theoretical capacity of a conventional lithium battery (140 mAh/g); (2) the sodium element as a cathode material, The amount of resources is much higher than that of lithium (the abundance of sodium in the crust and ocean (the Clark value) is more than 1,000 times that of lithium) and is easily available. However, when using a conventional electrolyte, Na-S batteries also have many problems, which limits their further development. The main problem is that when the battery is charged and discharged, the sulfur anode appears to dissolve into the electrolyte; the side reaction occurs inside the battery, thereby reducing the charge and discharge capacity; the battery has a short life and the like. When the ionic liquid is used as the electrolyte, the above problems are all suppressed. At the same time, the battery capacity and energy density parameters are also greatly improved compared with the conventional battery, and the battery cost is greatly reduced. Figure 6 uses an ionic liquid as an electrolyte to inhibit the dissolution of the anode of a Na-S battery. 5. Off-topics caused by ionic liquid batteries Throughout the process of using ionic liquids in batteries, we have found that even in the modern era of rapid technological development, basic science research is still very important. As history develops into the 21st century, as the pace of social development accelerates, people in the world become impetuous. Not only in basic research, but also in the application of technology, there are fewer and fewer people. It is difficult to feel in daily life: basic research is related to the “staying power†of a country and a nation, and the obscure basic research is the cornerstone that drives the application research and science and technology to make a leap. Before the 1990s, ionic liquids were only studied as a new type of liquid substance. The electrochemical performance of ionic liquids is not in the hands of people, and there is no objective requirement for applying these properties in society. Lead-acid batteries run through the history of the entire car and still occupy an important position in the storage components of automobiles. For conventional automotive systems powered by gasoline and diesel, the performance of lead-acid batteries is fully satisfactory (although it is not satisfactory in terms of its life). However, when environmental protection, energy conservation, light weight and new energy become the main theme of the automobile industry and the whole society, the shortcomings of lead-acid batteries are attracting more and more attention in the evaluation system of social values. The more efficient and environmentally friendly new batteries are getting more and more attention, and the requirements for the performance of the battery itself are getting higher and higher. When the development of the battery encounters a bottleneck, people naturally go back and look for new materials. This is one of the important reasons for the rapid development of ionic liquid research after the 1990s. Social needs are the driving force for the development of science and technology. However, if there is no basic research that the researchers have been down to on before, the world that is thriving now may be another way. Nearly 100 years ago, humans just entered the electrical age, and modern tungsten incandescent lamps (invented in 1906) have just entered people's lives. When is the New Zealand physicist Ernest? When Rutherford used the alpha ray to illuminate the nucleus, his purpose was simply to prove his theory of nuclear structure model. That is to say, at the time, the study of the deep structure of matter was a purely theoretical study of "high on the earth" that was completely unrelated to social life. However, just behind Rutherford’s research, there is a new, infinitely vast world hidden! Based on this experiment, people have developed a series of theories that change the world's appearance, such as atomic physics, quantum mechanics and relativity. In a sense, every aspect of our modern life benefits from the basic research that Rutherford did in the past! Mr. Robert Rathbun Wilson, former director of the Fermi NaTIonal Accelerator Laboratory in the United States, once had such an anecdote: when he proposed building a new high-energy physics accelerator When consulted by Congress, I was asked: Is the high-energy accelerator helpful for national defense? He replied: Building a high-energy accelerator is related to the dignity of our human beings and the worship of our culture by other countries... Although it has nothing to do with national defense, it will make our country worthy of defending! The importance of basic research is reflected in this story. Conclusion Ionic liquid batteries are a new technology developed in recent years, and all countries in the world are standing at the starting line of research and development. Therefore, it is easier to produce results when conducting research. At present, the research institutions of various countries are at the stage of exploration of relevant components. Combine the various ingredients to find effective ratios. What is needed in this research phase is not a profound theory, but a large number of matching experiments. Therefore, a large number of testers with basic skills are required. This is precisely the strength of our country that lacks basic researchers. As with other scientific studies, there is a process for the study of ionic liquids that translates from slow basic research into rapid applied research. In the era when the "fast food" culture has become the main theme of social life and people's consciousness, whether it is basic research or applied research, there is a question of how researchers and research institutions endure loneliness, hesitation and embarrassment before producing results. Now, China has entered the world's second largest economy. Under such circumstances, how to play a supporting role in research and development from related aspects such as the R&D system is related to how we continue to develop and win big questions in the world.
iPhone 4S Battery 3.7V Li-ion with 500 cycle life time which is make from brandnew
cell and high quality protection board and FPC,all the raw materials is customerized. 0
cycle and iPhone 4S Battery life more than 500 times, rejection rate under 3‰. We are professional manufacturer on iPhone battery.
Nominal voltage: 3.7V
Limited charge voltage: 4.2V
Capacity:1430mAh (5.3whr)
Cell size: 42x32x82mm
iPhone 4S Battery iPhone 4S Battery,iPhone battery,iPhone 4S Battery life,iPhone 4S Battery Pack Shenzhen Aokal Technology Co., Ltd. , https://www.aokals.com